SMART-LAB
Новый дизайн
Мы делаем деньги на бирже
Блог им. diamante
SRPT hot stock in play
- 03 октября 2012, 15:50
- |
Рост на премаркете больше, чем на 100%
Возможен шорт на фоне фиксации прибыли.
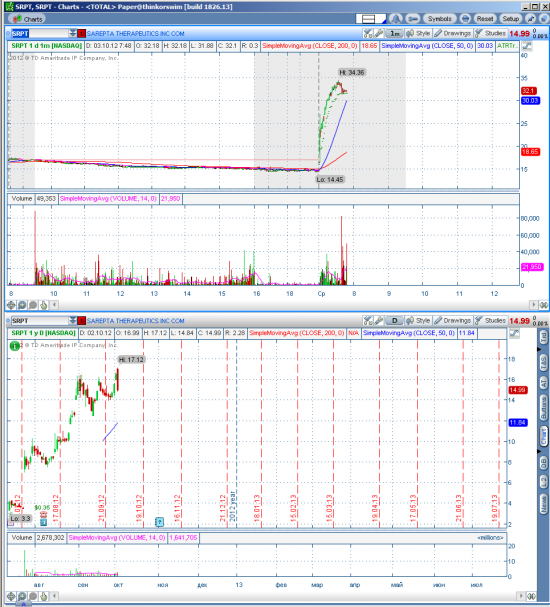
Вот и сама новость:
Sarepta Therapeutics announces eteplirsen meets primary endpoint of increased novel dystrophin and achieves significant clinical benefit on 6-minute walk test after 48 weeks of treatment in phase iib study in duchenne muscular dystrophy Price: 14.99 Change:
Co today announced that treatment with its lead exon-skipping compound, eteplirsen, met the primary efficacy endpoint, increase in novel dystrophin, and achieved a significant clinical benefit on the primary clinical outcome, the 6-minute walk test (6MWT) over the placebo/delayed treatment cohort in a Phase IIb extension trial in Duchenne muscular dystrophy (DMD) patients. Eteplirsen administered once weekly at either 30 mg/kg or 50 mg/kg for 48 weeks (n=8) resulted in a statistically significant increase (p=0.001) in dystrophin-positive fibers to 47.0% of normal. The placebo/delayed treatment cohort, which had received 24 weeks of eteplirsen at either 30 mg/kg or 50 mg/kg following 24 weeks of placebo (n=4), also showed a statistically significant increase in dystrophin-positive fibers to 38.3% of normal (p=0.009). Eteplirsen administered once weekly at 50 mg/kg over 48 weeks resulted in an 89.4 meter benefit compared to patients who received placebo for 24 weeks followed by 24 weeks of treatment with eteplirsen in the open-label extension. Co is holding a call at 8:00 ET to discuss.
Возможен шорт на фоне фиксации прибыли.

Вот и сама новость:
Sarepta Therapeutics announces eteplirsen meets primary endpoint of increased novel dystrophin and achieves significant clinical benefit on 6-minute walk test after 48 weeks of treatment in phase iib study in duchenne muscular dystrophy Price: 14.99 Change:
Co today announced that treatment with its lead exon-skipping compound, eteplirsen, met the primary efficacy endpoint, increase in novel dystrophin, and achieved a significant clinical benefit on the primary clinical outcome, the 6-minute walk test (6MWT) over the placebo/delayed treatment cohort in a Phase IIb extension trial in Duchenne muscular dystrophy (DMD) patients. Eteplirsen administered once weekly at either 30 mg/kg or 50 mg/kg for 48 weeks (n=8) resulted in a statistically significant increase (p=0.001) in dystrophin-positive fibers to 47.0% of normal. The placebo/delayed treatment cohort, which had received 24 weeks of eteplirsen at either 30 mg/kg or 50 mg/kg following 24 weeks of placebo (n=4), also showed a statistically significant increase in dystrophin-positive fibers to 38.3% of normal (p=0.009). Eteplirsen administered once weekly at 50 mg/kg over 48 weeks resulted in an 89.4 meter benefit compared to patients who received placebo for 24 weeks followed by 24 weeks of treatment with eteplirsen in the open-label extension. Co is holding a call at 8:00 ET to discuss.
теги блога day0markets.ru
- AMEX
- BAC
- Basic Materials
- BOVESPA
- charts
- CME
- Earning season
- earnings
- earnings season
- ECN
- ETF
- EWZ
- FDA
- floored
- Fusion
- halt
- HFT
- HLF
- Hotkeys
- ICE
- Imbalance trading
- inplay
- IQFeed
- machine learning
- Morgan Stanley
- multicharts
- NASDAQ
- NASDQ
- nflx
- NYSE
- python
- Sec fillings
- short float
- spin off
- SPY
- stocks in play
- stocks inplay
- STX
- takion
- thinkorswim
- trading floor
- UVXY
- VPS
- VRTX
- WDC
- азия
- акции
- алгоритмы
- алготрейдинг
- аналитика
- Бразилия
- Бразильская биржа
- брокеры
- бэктестинг
- волатильность
- выборы
- Гербалайф
- дауншифтинг
- дауншифтинг трейдера
- дивиденды в США
- заграница
- Индия
- инсайдеры
- исторические данные
- история котировок
- итоги года
- календарь отчетов
- Карл Айкон
- комиссии брокера
- корпоративные новости
- корреляция
- котировки NYSE
- криптовалюта
- лось
- Миграция
- ММВБ
- мысли о трейдинге
- новостной трейдинг
- опционы
- отбор акций
- офф топ
- оффтоп
- премаркет
- проп трейдинг
- раб место трейдера
- риск менеджмент
- Россия
- сезон отчетов
- сервер для робота
- серфинг
- статистика для трейдера
- такион
- торговая платформа
- торговые роботы
- трейдинг
- философия трейдинга
- фильм
- фьючерсы
- эмиграция
- юмор


















